Early detection of Alzheimer's
About Alzheimer’s Disease
Alzheimer's disease (AD) is a neurodegenerative disorder linked to genetic risk factors and characterized by memory loss and cognitive decline, particularly in older adults. While there is currently no cure, research has advanced in prevention strategies and therapies that relieve symptoms and slow disease progression.
Importance of Early Detection
Early diagnosis is essential for timely and effective interventions. Changes in AD biomarkers can appear years before clinical symptoms, highlighting the importance and feasibility of detecting the disease early.
Advances in Diagnostics
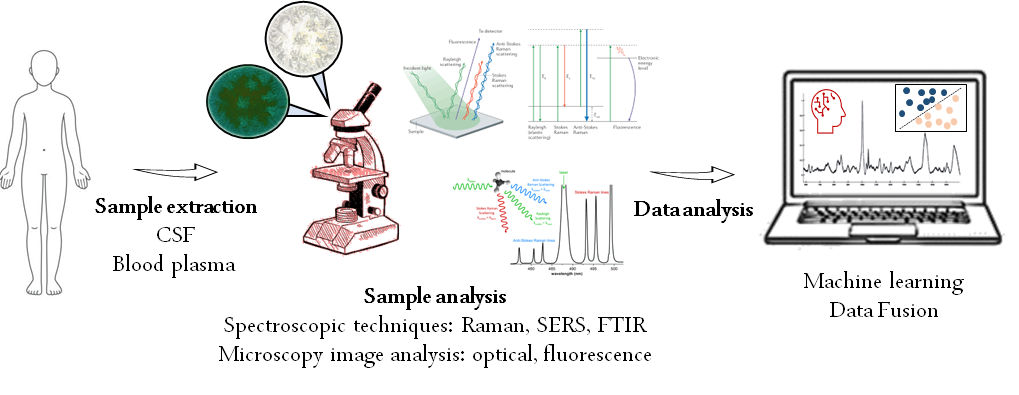
Detection methods for AD have progressed significantly, with increasing focus on early and accurate diagnosis. Biological fluids are commonly analyzed to detect AD biomarkers. Cerebrospinal fluid (CSF) analysis remains one of the most sensitive methods. However, recent efforts have prioritized blood and plasma-based tests, which are less invasive and more cost-effective.
Photonic techniques offer the unique ability to study biological samples in real-time without damaging them. Vibrational spectroscopy provides detailed chemical information, making it ideal for capturing the broad biochemical profile of these samples.
Our Project Approach
Building on these advancements, our project develops holistic analytical tools for in vitro diagnostics using machine learning. Plasma samples from patients at different disease stages and healthy controls are prepared following structured protocols and analyzed using microscopy and spectroscopic techniques such as Raman, surface-enhanced Raman (SERS), and Fourier-transform infrared (FTIR) spectroscopy.
Unlike conventional methods that measure biomarkers directly, our approach detects subtle changes in plasma morphology as indicators of disease. This work is carried out by a four-member consortium, including CIC nanoGUNE (organizer), CITA-Alzheimer, Vicomtech, and the University of the Basque Country UPV/EHU (IBeA).
Publications
, , , , , , , , , “Hyperspectral Raman imaging to understand patterns in dried biofluids in Alzheimer’s disease,” VIEW, vol. 6, no. 2, Art. e20250034, 2025.
J. Bóbeda et al. Alzheimer’s Disease Classification by Artificial Intelligence Using Microscopy Images of Dried Human Body Fluids. In: Chen, YW., Tanaka, S., Howlett, R.J., Jain, L.C. (eds) Innovation in Medicine and Healthcare. KES InMed 2024. Smart Innovation, Systems and Technologies, vol 412. Springer, Singapore. https://doi.org/10.1007/978-981-97-7498-2_6
J. Etxebarria-Elezgarai et al., “Surface-enhanced Raman spectroscopy for early detection of Alzheimer’s disease,” in Proc. 2024 Int. Conf. on Optical MEMS and Nanophotonics (OMN), IEEE, pp. 1–2, 2024, doi: 10.1109/OMN61224.2024.10685286.
E. Lopez, J. Etxebarria-Elezgarai, M. García-Sebastián, M. Altuna, M. Ecay-Torres, A. Estanga, M. Tainta, C. López, P. Martínez-Lage, J. M. Amigo, and A. Seifert, “Unlocking preclinical Alzheimer’s: A multi-year label-free in vitro Raman spectroscopy study empowered by chemometrics,” International Journal of Molecular Sciences, vol. 25, no. 9, p. 4737, 2024.
